KARACHI: In a landmark public health decision, the Drug Regulatory Authority of Pakistan (DRAP) has officially suspended the marketing and use of the hormonal drug 17-Hydroxyprogesterone Caproate (17-OHPC) commonly marketed in Pakistan as Gravibinan citing serious cancer risks in unborn children.
This decisive action comes after two years of delay, with previous administrations hesitant to act amid pressure from powerful pharmaceutical interests. However, the newly appointed DRAP Chief Executive has swiftly resolved the matter within one month of taking office, signaling a strong commitment to public safety over corporate influence.
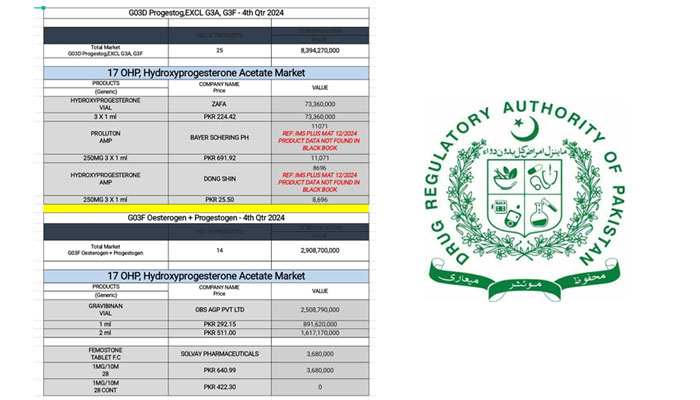
According to official records from the 347th Registration Board Meeting agenda, the suspension follows recommendations of the Pharmacovigilance Risk Assessment Expert Committee (PRAEC), based on international assessments by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The data show that exposure to 17-OHPC during pregnancy carries a proven risk of cancer in children later in life, while its benefits in preventing preterm birth remain unsubstantiated.
> “This bold step aligns Pakistan with global regulatory standards. The benefit-risk ratio of 17-OHPC is no longer in favor of its continued use,” a senior DRAP official stated.
The injection, used primarily to prevent preterm labor in high-risk pregnancies, had already been withdrawn in the US and Europe, but remained available in Pakistan under the brand Gravibinan — with an estimated market worth of over PKR 3 billion annually.
Industry Reaction: Resistance vs. Reform
Pharmaceutical stakeholders have privately expressed concerns over the abrupt nature of the decision, citing potential disruptions in maternal care and ongoing clinical reliance. However, public health experts have lauded DRAP’s firm stance as a turning point for ethical drug regulation in Pakistan.
> “This isn’t just a drug safety issue — it’s about restoring trust in Pakistan’s regulatory system,” remarked a public health analyst. “For too long, harmful drugs have lingered in the market due to lobbying and delays. This decision sets a precedent.”
The new DRAP leadership’s refusal to yield to corporate pressure has drawn public praise and sparked hope for a new era of transparency and accountability in Pakistan’s health sector.
DRAP has instructed its Registration Board to ensure immediate implementation of the suspension, directing that all stocks of 17-OHPC be withdrawn from the market. Healthcare providers are advised to cease prescribing the drug and explore alternative treatment options as guided by updated clinical protocols.
Health Matters Media will continue to monitor this developing story, including the impact on clinical practice and patient outcomes across Pakistan.